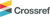Particle Scavenging Properties of Rain Clarified by a Complementary Study with Bulk and Semi-bulk Samples
Copyright © 2018 Korean Society for Atmospheric Environment
Abstract
It is a well-known fact that precipitation plays an important role in capturing ambient particles, however, the details of particle scavenging properties have not been fully proved. To clarify the particle scavenging properties, a complementary study was carried out with the bulk and semi-bulk rain samples collected in an urban city of Japan. pH showed a continued downturn for a little bit at the beginning rainfall and then a turn-up in the following rainfall. The recorded pH values of rainwater (ranged from 3.5-4.6) demonstrated that the strong acid rain was observed during our field measurements. Compared to the subsequent rainfall, electrical conductivity in the beginning rainfall had about 1.3 times higher level. Sulfur showed an overwhelmingly high concentration compared to other elements in both ambient total suspended particles (TSP) and rain samples. On the contrary to ambient TSP, every element including Ca and Zn in rain showed a continued rise in concentration accompanied by increasing of rainfall amount. During the first period of the rainfall there was no meaningful change in elemental carbon concentration, however, it was largely increased (up to 0.2 mg L-1) in the sequential rainfall (4.0-4.5 mm rainfall amount). The theoretically calculated number concentration of particles scavenged by raindrops showed a strong decrease of with the increasing droplet diameter regardless of particle type.
Keywords:
Raindrop, Washout, Particle scavenging, Elemental carbon, Acid rain, Residual particle1. Introduction
Atmospheric particulate matters are removed by wet and/or dry depositions, the former proceeds more efficient removal in the form of precipitation such as rain and snow (Byrne and Jennings, 1993). A clear view of the sky and landscapes after rainfall is a good example to understand that rain is a great cleaner of ambient particulate matters.
It can be therefore thought that there are three major components to the potential impact of the atmospheric particulate matter on human health. They are the pre-depositional phase, depositional phase, and post-depositional phase. The pre-depositional phase is direct human exposure to toxic and acidic substances from ambient air because soluble particles depositing anywhere of our respiratory system can dissolve and release the potentially harmful material to the body. Depositional phase is probably caused by the exposure of rain incorporating a large amount ambient harmful substance. Meanwhile, the post-depositional phase can be caused by the influx of harmful substances through contaminated soil and underground water (Goyer et al., 1985).
The incorporation of ambient particles into raindrops mainly occurred by the collision between particles and falling raindrops. The efficiency of the collision between particles and falling raindrops depends on their size distributions (Byrne and Jennings, 1993). The amount of pollutants scavenged by rain is also variable depend on rainfall properties (e.g., rainfall amount, rainfall duration, rainfall intensity, and raindrop size distribution). The study of chemical components in size-resolved raindrops realized by the collection of raindrops as a function of their size (Ma et al., 2004; Tenberken and Bächmann, 1996) suggested that among various rainfall properties, raindrop size distribution is crucially important because it plays an important role in capturing pollutants.
Ma and Sera (2017) reported that in the assessment of public health risks associated with ambient particulate matter, especially PM2.5, a study of the chemical nature of the precipitation is no less important than that of the ambient particulate matter itself.
In order to fully understand the wet scavenging properties of ambient particles and those wet deposition amounts, the chemical characteristics of both ambient particle and the rain sample collected simultaneously have to be specified. For this, our complementary study has been carried out with the bulk and semi-bulk rain samples collected in an urban city of Japan
2. Experimental Methods
2. 1 Collection and Handling of Bulk and Semi-bulk Rain Samples
To collect bulk rainwater the rain samplers with a polyethylene funnel (inner diameter 30 cm) mounted on an iron tripod and a polyethylene bottle (a capacity of 500 ml) connected to the funnel by means of a screwcap.
For the sampling of semi-bulk rain sample (i.e., separated rainwater as a function of raindrop size), the raindrop collector devised by our oneself in previous research (Ma et al., 1999) was applied. Details on the principle of our own raindrop collector can be found in our previous papers (Ma et al., 2004).
Two kinds of rain collectors were located on the rooftop of a four-story building (a height of 20 m above ground level) (33.40 N 130.26 E) at Fukuoka Women’s University in Fukuoka City of Japan just before the rain. In order to reasonably clarify the particle scavenging properties of rainfall, an ideal continuous rain over the middle to the end of March 2014 was the target of our intensive field measurements.
Collection of both bulk and semi-bulk rain was taken six times according to the rainfall amount. The rain event lasted 14 h, with rain intensity of 0.2-1.2 mm h-1, and air temperature between 9.6-16.4℃.
2. 2 Sampling of TSP
For the sampling of total suspended particles (TSP), a filter pack sampler (Tokyo Dylec Co.) was operated near the rain collection site. TSP was collected on a 47 mm diameter Nucleporefilter® placed on the impaction plate of a filter pack sampler. In our experiment, a filter pack sampler was operated for four times according to the rainfall amount.
2. 3 Pretreatment of Bulk and Semi-bulk Rain Samples
After sampling, pH and electrical conductivity were determined immediately in bulk and semi-bulk rain samples. And then both samples were agitated with an ultrasonicator. After agitating, some of rain samples were filtrated through a 25 mm diameter Nuclepore® filter with 0.2 μm pore size to separate into the soluble and insoluble fractions. Then, the filtrates were the targets of ionic analysis. A 20 μL of filtrate was dropped on a non-hole Nuclepore® filter and dried by an infrared lamp. And then, they were the targets of elemental analysis. Meanwhile, some other rain samples were filtrated through a 25 mm quartz fiber filter. Because there is no current standardized analysis for measuring elemental carbon concentration in precipitation, the insoluble wet particle-laden quartz fiber filters were dried in a desiccator for 24 h (30°C, 55% relative humidity) and become the target of the determination of elemental carbon. Fig. 1 illustrates the flow of sampling, pretreatment, and analysis of bulk and semi-bulk rain samples.
2. 4 Chemical Analyses
Elemental analysis for the soluble fraction of rain samples was subsequently analyzed by Particle Induced X-ray Emission (PIXE). The PIXE installed at the Cyclotron Research Center of Iwate Medical University was applied and it has the great advantages such as an excellent sensitivity, a nondestructive technique for multielement with a wide range of elements (Z>10). The sensitivity, if defined by the ratio between PIXE yield per unit dose and mass thickness, can be determined for all objective elements both experimentally and theoretically. For instance, the sensitivity of calcium was calculated to be 1700 (counts· cm2 (μC·μg)-1) with a detection limit of 9.4×10-3 (μg cm-2). The more detailed analytical procedures and experimental setup for PIXE analysis were described elsewhere (Sera et al., 1999).
Ion Chromatography (IC) (Dionex, DX-100) was used to determine the concentrations of various ions present in both rain samples. Samples were analyzed for both cations (sodium, ammonium, potassium, magnesium, and calcium) and anions (fluoride, chloride, nitrate, phosphate, and sulfate). The more detailed analytical set-up and the procedures on sample pretreatment for IC analysis is given elsewhere (Kang, 2003).
The concentration of elemental carbon was determined using the TOR® (DRI) Method. Two 0.64 cm2 punches were taken from the quartz-fiber filter used the filtration of raw rain sample and placed in the analyzer. In analytical processes, elemental carbon is removed from quartz-fiber filter capturing the insoluble particles by volatilization, and/or combustion at selected temperatures (550, 700, and 800℃ for 150- 580 s), and by conversion of the released gases to CO2 or CH4, followed by infrared absorption (CO2) or flame ionization (CH4) detection (Chow et al., 1993). This TOR® method is a well-accepted technique in which the sample is progressively pyrolyzed with continuous detection of evolved carbon. Chow et al. (1993) gave a full detail of TOR® method.
2. 5 Number Concentration Monitoring of Size-resolved Particles
The number concentrations of size-selective particulate matters (i.e., 0.3-0.5 μm, 0.5-1.0 μm, 1.0-2.0 μm, and 2.0-5.0 μm) were also monitored by an optical particle counters (OPC) (RION, KC-01D).
The overall process of collection and handling of size-resolved raindrops and TSP, and their chemical analyses is shown in Fig. 1.
3. Results and Discussion
3. 1 Physical Properties of Bulk and Semi-bulk Rain Samples
The electrical conductivity of rain sample is referred as one of key factors for determining how much pollutants exist in rain sample. Although, electrical conductivity in a solution can be simply measured by several types electrical conductivity meter on the market, it was also theoretically calculated by following Kohlrausch’s law expressed as a weighted sum of the equivalent conductivity of each ionic species (Appelo, 2010).
This equation states that the equivalent conductivity of an electrolyte at infinite dilution is equal to the sum of the conductances of the anions and cations.
where NIon is total number of ions and it is 11 because a total of 11 ionic species (chloride, nitrate, sulphate, phosphate, bicarbonate, sodium, ammonium, potassium, magnesium, calcium, and hydrogen) was specified in this study. The quantities Vi, Mi, and Ci are the charge number of ion i, molar concentration (mol cm-3) of ion i, and the value of limiting molar conductivities (S cm2 mol-1) at 25°C of ion i, respectively.
ECcal., as mentioned above, is basically based on the molar concentration of ionic species determined by IC analysis. This means if the ions are not analyzed properly the theoretical evaluation of EC cannot produce a successful outcome. In addition, through the comparison of ECcal. with the measuring results of electrical conductivity by electrical conductivity meter (Shodex CD-200), it is possible to practice the QA of IC analysis data. The measurement range and limit of resolution of electrical conductivity meter are 0.0025~51.2 mS m-1 and 0.01 mS m-1, respectively.
In this study, a t-test was conducted to determine whether the means of two data set (i.e., ECcal. and the measured electrical conductivity), which were designed by analyzing/calculating of six rain samples collected as a function of rainfall amount, are statistically different from each other. The null hypothesis for t-test was that the means of the paired independent samples were homogenous. As the result of t-test, the null hypothesis cloud not be rejected. There was not a significant difference in the scores for ECcal. (mean=56, standard deviation=21.28) and the measured EC (mean=59, standard deviation=22.06) conditions; t(6)= - 2.36, p=0.142. This indicates that the two means were not statistically different from each other and suggests that ion analysis was reliable.
Fig. 2 shows the variation of pH of bulk rainwater measured at right after rain sampling as a function of rainfall amount and the ECcal. mainly based on the results of ion analysis. For the measurement of the pH of rain samples, a pH meter (JIS Z8802) with the sensitivity less than±0.005 was employed.
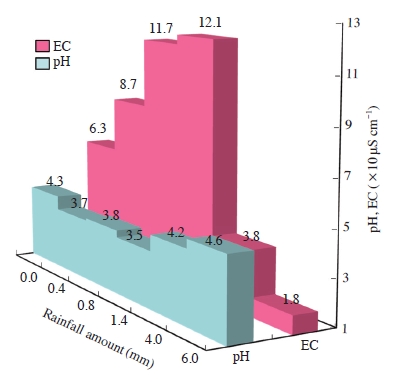
Variation of pH and electrical conductivity (EC) of bulk rainwater as a function of rainfall amount.
pH represents a gradual change between 3.5 and 4.6. It shows a downward trend in the initial rain (rainfall amount from 0.0 to 1.4 mm), but it rose again. The rain fallen during a whole period of measurement was acid rain with a pH level was far lower than 5.6. The pH fluctuation range of this study is different compared to those measured at two different urban areas, i.e., Chongqing in China and Kawasaki in Japan. Although, 76.2% of all rain samples had a pH of less than 5.0, the pH of rainwater measured in Chongqing known as the largest industrial and economic center in southwestern China was in the range from 3.8-7.6. In the case of Kawasaki, which has been the basic engine of Japan’s economic development for decades, the pH varied from 3.8-6.6 and the rain with pH below 5.0 was 75.1% (Lu and Yagoh, 2007).
Meanwhile, unlike pH, the electrical conductivity of bulk rainwater shows a large fluctuation raged from 18 to 121 μS cm-1 according to rainfall amount. In the case of early rainfall (beginning rainfall), it is seen to increase with an increase in rainfall amount. However, a sharp falling of electrical conductivity was shown in the following rainfall (subsequent rainfall) from 4.0 to 6.0 mm rainfall amount.
In the study of rainwater in Karachi, which is the largest and most populous city in Pakistan, carried out by Chughtai et al. (2014), the conductivity in Karachi rainwater samples ranged between 20-210 μS cm-1. In the case of the rain samples collected at the mountain area located in South America, electrical conductivity was relatively low and varied from 3 to 11 μS μS cm-1 (Beiderwieden et al., 2005).
To know how electrical conductivity varies according to the size of raindrops will be a very meaningful thing for assessing the impact of fine rain and showers on ecosystem. Table 1 summarizes electrical conductivity and pH of rainwater as the functions of raindrop size and rainfall amount. Electrical conductivity of the small raindrops (<0.17 mm) was 140 μS cm-1 and it was about three or five times higher than those of relatively large raindrops. Compared to the subsequent rainfall, electrical conductivity in the beginning rainfall had about 1.3 times higher level. Meanwhile, although an extreme shift was not shown among the pH of three-step raindrop sizes collected both beginning and subsequent rainfalls, it varied from 4.0 to 5.2.

Electrical conductivity (EC) and pH of rainwater as the functions of raindrop size and rainfall amount.
The electrical conductivity is referred as one of key factors for determining the amount of water soluble electrolytes retained in rainwater that depends on cloud formation (rain out) and wet-scavenging (wash out) processes.
It can also be thought that inorganic ions as well as organic acids affect the acidity and electrical conductivity of rainwater. Galloway et al. (1982) reported that the potential contributions of organic anions to the free acidity of rain was about 60%. Similar result has been observed in the Amazon rain forest at Manaus, Brazil (Andreae et al., 1988).
3. 2 Variation of Chemical Components According to Rainfall Amount
Fig. 3 shows the concentrations of several interested elements (S, Ca, Zn, Cl, and Na) in TSP and rain. Sulfur shows an overwhelmingly high concentration compared to other elements in both ambient TSP and rain samples. Despite the tiny changes in rainfall, in most of the elements, concentrations were fluctuating sharply for a period of short initial rainfall. As typical water-soluble components, S, Cl, and Na shows a marked reduction in accordance with rainfall amount. Meanwhile, the concentration of Ca and Zn indicate an even consistency regardless of rainfall amount. On the contrary to ambient TSP, every element including Ca an Zn in the rain shows a continued rise in concentration accompanied by changes in rainfall amount.
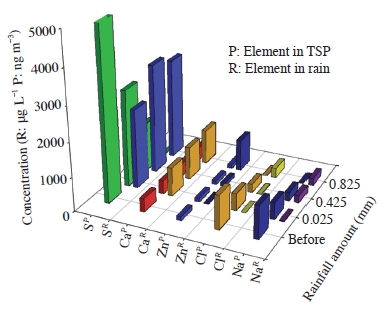
Variation of several interested elements in TSP and bulk rain samples collected before and during an initial rainfall event.
As mentioned above, electrical conductivity of rainwater was also drastically increased according to rainfall amount during an initial rainfall event. This result, therefore, was probably caused by the increasing of electrolyte concentration in rainwater through a below-cloud scavenging of air pollutants.
Estimation of wet deposition of elemental carbon is crucial for the understanding of its circulation between surface and troposphere, lifetime, and radiative forcing. Till now, there are limited data available concerning the elemental carbon concentration in bulk rain samples (Hwang and Ma, 2003; Jennings et al., 1997). Not to mention, there is no precedent for the raindrop size dependence of the elemental carbon concentration probably due to specific problems of collection and handling of size-resolved raindrops. Unfortunately, the physical and chemical parameters related to wet deposition are not well understood because wet deposition is a complex multiphase system.
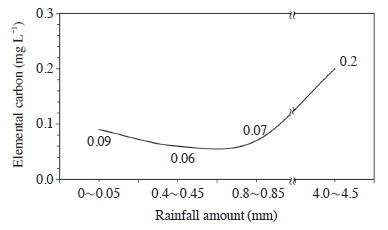
Fig. 4 shows the variation of elemental carbon in rainwater as a function of rainfall amount. Elemental carbon concentration varied from 0.09 to 0.2 mg L-1. During the first period of the rainfall (between rainfall amount 0 to 0.85 mm), there was no observable change in elemental carbon concentration. Meanwhile, a definite change in the sequential rainfall (4.0-4.5 mm rainfall amount) occurred, namely elemental carbon concentration was largely increased (up to 0.2 mg L-1).
Until about 20 years ago, it was widely understood that elemental carbon does not form cloud droplet. However, relatively recently, Hitzenberger et al. (1999) and Dusek et al. (2006) reported that the CCN activation of elemental carbon through their laboratorial and field experiments. More recently, Ma and Kim (2014) have visually and chemically demonstrated the CCN activation of soot particles from their laboratory-scale model experiment. The results of these laboratorial and field experiments suggest that the elemental carbon existed during the initial rain in this study might be mainly driven from its CCN (cloud condensation nuclei) activation.
Freshly emitted below cloud elemental carbon is insoluble in water (Zuberi et al., 2005). However, it can be changed to perfect wettable (hydrophilic) if it has experienced a chemical modification process and then water vapors spread out on its surface in the high humid atmosphere (Ma et al., 2004). The hydroscopic aged elemental carbon containing aerosols can be transferred into falling raindrops. Hwang et al. (2004) reported that a large amount of soot particles suspended in the air could be removed by precipitation scavenging mechanisms.
Therefore, the increasing of elemental carbon concentration during a following rain fall (4.0-4.5 mm rainfall amount) might be caused by the washout of the chemically modified hydroscopic EC and hydrophilic aged elemental carbon containing aerosols.
3. 3 Theoretical Calculation of the Scavenged Particle Number in Size-resolved Raindrops
In our previous study (Ma and Sera, 2017), the collection efficiency of ambient particles for three kinds of raindrop sizes (i.e., 0.09, 0.94, and 2.60 mm diameter) was theoretically computed using the particle collection efficiency (Einteg.) of raindrops. Einteg. integrated three kinds efficiencies, i.e., E by Brownian diffusion (Edif.), E by interception (Eint.), and E by inertial impaction processes (Eimp.). Details for model calculation including variable settings and computation processes were already described in our earlier articles (Ma et al., 2004).
In this study, furthermore, a theoretical calculation of the particle number scavenged in three-step of raindrop size spectra was newly attempted. Three kinds of major secondary particles (i.e., NH4NO3, NH4Cl, and (NH4)2SO4) were the target of model calculation.
In order to perform a successful model calculation, following factors are needed.
- - scavenging cross section of each size of raindrop (dr), π (dr/ 2)2
- - terminal velocity of raindrop according to raindrop size, υt (dr) (m sec-1)
- - ambient mass concentration of each particle type at ground, Cp,0 (dp) (μg m-3)
- - particle (dp) scavenging efficiency of a raindrop (dr), E0 (dr, dp)
- - mass per a drop for one second (mp), μg dr-1sec-1
- - mass of particles (dp) in a raindrop (dr) over all t per a drop falling from cloud height (hc) (Mp), μg dr-1
- - particle (dp) number retained in a whole raindrop, Np, drtotal-1
Cp,0 (dp) (μg m-3) of three kinds secondary particles were stoichiometrically determined from the ionic concentration (nitrate, chloride, and sulfate) of PM2.5 measured at Fukuoka by Fukuoka City during our field campaign period (Composition and sources of PM2.5 in Fukuoka, 2014).
When a raindrop with diameter dr falls for one second, the volume (V) swept by a falling raindrop is described as following equation:
where υt is the terminal velocity of a raindrop.
For model calculation, we assumed that particles were uniformly distributed in V.
The mass (mp) of particle (dp) in raindrop (dr) (μg dr-1s-1) falling through V for one second can be calculated by following equation:
Therefore, the total mass (Mp) of particle (dp) in raindrop (dr) falling through from below cloud base to ground can be constructed as following equation:
where hc is specified by the weather information of the Japanese Meteorological Agency on the day of field measurement.
Table 2 summarizes the parameters required to model calculation, the calculated mp, and Mp.
Finally, Np in three-step of raindrop size spectra cloud be calculated using the following equation:
where δdp is the densities of NH4NO3, NH4Cl, and (NH4)2SO4.
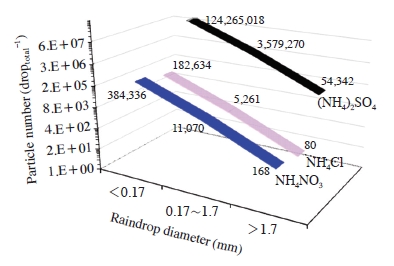
Fig. 5 shows the theoretically calculated number concentration of particles retained in a whole raindrop in each raindrop size. As shown in Fig. 5, the particle number of three major secondary particles scavenged by raindrop was found to be strong raindrop size dependence. It showed a strong decrease of the number concentration of scavenged particles with increasing droplet diameter regardless of particle type. Although this is the calculated result, (NH4)2SO4 captured by raindrops shows a predominantly higher number concentration in whole raindrop size spectra. Despite the secondary sulfate particles (mainly (NH4)2SO4 or NH4HSO4) are water soluble, their size is generally appeared in the fine mode at 0.5-0.6 μm (Taiwo et al., 2014). In general, as first suggested Greenfield (1957), there is a minimum collection efficiency (i.e., the Greenfield gap) for the particle radius near 0.1 μm. Therefore, particles with radii less than about 0.1 μm and the much larger particles than the Greenfield gap boundaries are more efficiently scavenged in raindrops due to their Brownian motion and their inertia, respectively (Croft et al., 2009).
4. Conclusions
In the present study, the fact that ambient particles are efficiently removed from the atmosphere by wet precipitation has been clarified by a complementary study with the bulk and semi-bulk rain samples collected in an urban city of Japan. Typical water-soluble components such as S, Cl, and Na showed a marked reduction in accordance with rainfall amount. As one of unexpected facts, even though elemental carbon is insoluble in water, there was its observable removal in the sequential rainfall. The theoretical calculation of the scavenged particle number in three-step of raindrop size spectra was newly attempted in the present study. A new information that three kinds of major secondary particles (i.e., NH4NO3, NH4Cl, and (NH4)2SO4) captured by raindrops showed a high number concentration in whole raindrop size spectra was apparently discovered in this study. It can be said that the analysis of single and size-classified raindrops in this study is expected to give new and interesting information about wet scavenging of anthropogenic air pollution such as elemental carbon and the secondary particulate matters. In terms of health hazards of ambient particulate matters, new information obtained through our first experimental and theoretical evaluations for the particle scavenging properties of rain should be welcomed by both health people and people with compromised health, particularly people living in East Asia, who suffer from PM2.5.
Acknowledgments
This paper was supported by Wonkwang Health Science University in 2017. The authors wish to express thanks to Prof. Koichiro Sera at Cyclotron Research Center, Iwate Medical University for his help of PIXE analysis.
References
-
Andreae, M.O., Talbot, R.W., Andreae, T.W., Harris, R.C., (1988), Formic and acetic acid over the central Amazon region, Brazil: 1. Dry season, Journal of Geophysical Research, 93(D2), p1616-1624.
[https://doi.org/10.1029/jd093id02p01616]
- Appelo, C.A.J., (2010), Specific conductance-how to calculate the specific conductance with PHREEQC, http://www.hydrochemistry.eu/exmpls/sc.html.
-
Beiderwieden, E., Wrzesinsky, T., Klemm, O., (2005), Chemical characterization of fog and rain water collected at the eastern Andes cordillera, Hydrology and Earth System Sciences, 9, p185-191.
[https://doi.org/10.5194/hess-9-185-2005]
-
Byrne, M.A., Jennings, S.G., (1993), Scavenging of sub-micrometer aerosol particles by water drops, Atmospheric Environment, 27A(14), p2099-2105.
[https://doi.org/10.1016/0960-1686(93)90039-2]
-
Chughtai, M., Mustafa, S., Mumtaz, M., (2014), Study of physicochemical parameters of rainwater: A case study of Karachi, Pakistan, American Journal of Analytical Chemistry, 5, p235-242.
[https://doi.org/10.4236/ajac.2014.54029]
-
Chow, J.C., Watson, J.G., Pritchett, L.C., Pierson, W.R., Frazier, C.A., Purcell, R.G., (1993), The DRI thermal/optical reflectance carbon analysis system: description, evaluation and applications in U.S. air quality studies, Atmospheric Environment, 27A(8), p1185-1201.
[https://doi.org/10.1016/0960-1686(93)90245-t]
-
Croft, B., Lohmann, U., Martin, R.V., Stier, P., Wurzler, S., Feichter, J., Posselt, R., Ferrachat, S., (2009), Aerosol size-dependent below-cloud scavenging by rain and snow in the ECHAM5-HAM, Atmospheric Chemistry and Physics, 9, p4653-4675.
[https://doi.org/10.5194/acpd-9-7873-2009]
-
Dusek, U., Reischl, G.P., Hitzenberger, R., (2006), CCN activation of pure and coated carbon black particles, Environmental Science and Technology, 40(4), p1223-1230.
[https://doi.org/10.1021/es0503478]
- Fukuoka City, (2014), Composition and sources of PM2.5 in Fukuoka. Research report of Fukuoka City, p1-4.
-
Galloway, J.N., Likens, G.E., Keene, W.C., Miller, J.M., (1982), The composition of precipitation in remote areas of the world, Journal of Geophysical Research, 87(C11), p8771-8786.
[https://doi.org/10.1029/jc087ic11p08771]
-
Goyer, R.A., Bachmann, J., Clarkson, T.W., Ferris, B.G., Graham, Jr. J., Mushak, P., Perl, D.P., Rall, D.P., Schlesinger, R., Sharpe, W., Wood, J.M., (1985), Potential human health effects of acid rain: report of a workshop, Environmental Health Perspectives, 60, p355-368.
[https://doi.org/10.1289/ehp.8560355]
-
Greenfield, S.M., (1957), Rain scavenging of radioactive particulate matter from the atmosphere, Journal of Meteorology, 14, p115-125.
[https://doi.org/10.1175/1520-0469(1957)014<0115:rsorpm>2.0.co;2]
-
Hitzenberger, R., Berner, A., Giebl, H., Kromp, R., Larson, S.M., Rouc, A., Koch, A., Marischka, S., Puxbaum, H., (1999), Contribution of carbonaceous material to cloud condensation nuclei concentrations in European background (Mt. Sonnblick) and urban (Vienna) aerosols, Atmospheric Environment, 33(17), p2647-2659.
[https://doi.org/10.1016/s1352-2310(98)00391-4]
- Hwang, K.C., Ma, C.J., (2003), The distribution characteristics and long-term trend of carbonaceous species in airborne particulate in Seoul between 1986 and 1996, Journal of Korea Society for Atmospheric Environment, 19(E1), p11-20.
- Hwang, K.-C., Ma, C.-J., Cho, K.-C., (2004), Scavenging property of atmospheric carbon by precipitation, Journal of Korea Society for Atmospheric Environment, 20(E2), p77-85.
-
Jennings, G.S., Geever, M., McGovern, F.M., Francis, J., Spain, G., Donaghy, T., (1997), Micro-physical and physico-chemical characterization of atmospheric marine and continental aerosol at Mace Head, Atmospheric Environment, 31, p2795--2808.
[https://doi.org/10.1016/s1352-2310(97)00039-3]
- Kang, G.U., Shin, D.Y., Kim, H.K., (2003), Analysis of precipitation chemistry at rural site in the eastern coast, Korea, Journal of Korean Society for Atmospheric Environment, 19(E1), p29-39.
- Lu, Y., Yagoh, H., (2007), Comparison of air pollution and acid deposition between two mega-cities, EANET Research Fellowship Program, p57-76.
- Ma, C.-J., Kasahara, M., Hwang, K.C., Choi, K.C., Kim, H.K., (1999), Measurement of the single and size-classified Raindrops, Journal of Korea Society for Atmospheric Environment, 15E, p73-79.
-
Ma, C.-J., Kim, K.-H., (2014), Preliminary study on the cloud condensation nuclei (CCN) activation of soot particles by a laboratory-scale model experiments, Asian Journal of Atmospheric Environment, 8(4), p175-183.
[https://doi.org/10.5572/ajae.2014.8.4.175]
-
Ma, C.-J., Sera, K., (2017), The chemical nature of individual size-resolved raindrops and their residual particles collected during high atmospheric loading for PM2.5, Asian Journal of Atmospheric Environment, 11(3), p176-183.
[https://doi.org/10.5572/ajae.2017.11.3.176]
-
Ma, C.-J., Tohno, S., Kasahara, M., Hayakawa, S., (2004), Determination of the chemical properties of residues retained in individual cloud droplets by XRF microprobe at SPring-8, Nuclear Instruments and Methods in Physics Research B, 217(4), p657-665.
[https://doi.org/10.1016/j.nimb.2003.12.042]
-
Sera, K., Futatsugawa, S., Matsuda, K., (1999), Quantitative analysis of untreated bio-samples, Nuclear Instruments and Methods in Physics Research B, 150(1-4), p226-233.
[https://doi.org/10.1016/s0168-583x(98)01071-4]
-
Taiwo, A.M., Beddows, D.C., Shi, Z., Harrison, R.M., (2014), Mass and number size distributions of particulate matter components: comparison of an industrial site and an urban background site, Science of the Total Environment, 475, p29-38.
[https://doi.org/10.1016/j.scitotenv.2013.12.076]
-
Tenberken, B., Bächmann, K., (1996), Analysis of individual raindrops by capillary zone electrophoresis, Journal of Chromatography A, 755(1), p121-126.
[https://doi.org/10.1016/s0021-9673(96)00572-9]
-
Zuberi, B., Johnson, K.S., Aleks, G.K., Molina, L.T., Molina, M.J., Laskin, A., (2005), Hydrophilic properties of aged soot, Geophysical Research Letters, 32, pL01807.
[https://doi.org/10.1029/2004GL021496]